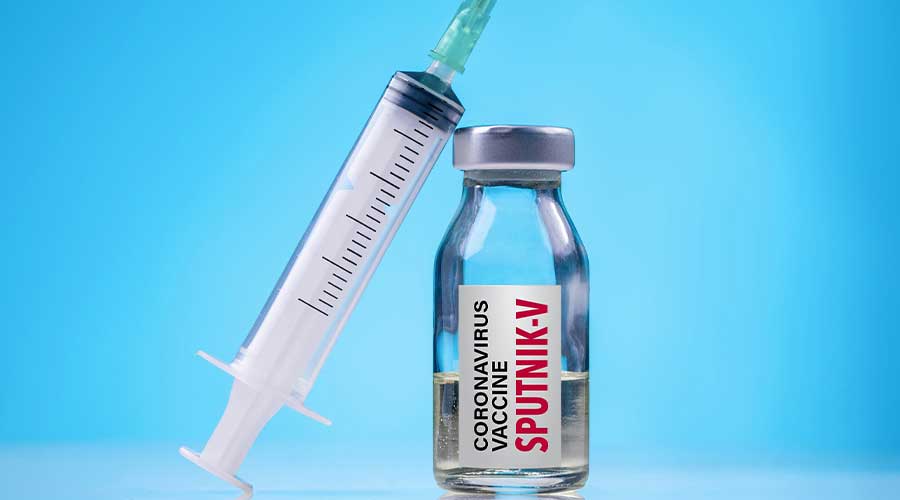
The Drug Regulatory Authority of Pakistan (Drap) has granted approval for the emergency use of the Russian-developed Sputnik V coronavirus vaccine. DRAP expects the first consignment of the vaccine to reach Pakistan by January end.
Drap has cleared and recommended the vaccine to the drug registration board, said Akhtar Abbas Khan, spokesperson for the Drug Regulatory Authority of Pakistan.
Pakistan as the world’s fifth most-populous nation is looking to start inoculations against the coronavirus.
Pakistan has already ordered 1.2 million doses of Chinese Sinopharm vaccine, with deliveries to start 31 January. Moreover, CanSino Biologics Inc. has also offered 20 million shots to Pakistan.
Last week, the authority had approved Oxford–AstraZeneca Covid vaccine that may cost around $6 in Pakistan. A private firm has been allowed to import the vaccine in the country.
Besides approving AstraZeneca and Sputnik V vaccines, the Drug Regulatory Authority of Pakistan (Drap), On Monday, had also approved Chinese state-owned firm Sinopharm’s Covid-19 vaccine for emergency use while the second shot is expected to be given approval for use in the country.
The spokesperson for the regulatory body said in a statement, “In a meeting conducted by [the] Registration Board of Drap today January 18, 2021, another vaccine manufactured by China National Pharmaceutical Group (Sinopharm) has also been given EUA (emergency use authorisation).”
“Both the Oxford and Sinopharm vaccines were evaluated for their safety and quality and granted EUA “with certain conditions”, the statement added, “This authorisation will be reviewed on quarterly basis keeping in view further data regarding safety, efficacy and quality.”
This approval allows federal and provincial governments as well as the private sector to import the vaccine from China.
The Beijing Institute of Biological Products, a subsidiary of state-owned conglomerate Sinopharm developed the vaccine. Preliminary data from last-stage trials shown to be 79.3 percent effective, announced the company last month when Chinese health regulators gave conditional approval to the Sinopharm vaccine, the first to be approved for general use in China.