United Kingdom turn out to be the first country to support an anti-Covid pill, as it allowed the use of Merck’s antiviral medicine for treating patients suffering from mild to moderate coronavirus, regulators said.
According to health minister Sajid Javid, “Today is a historic day for our country, as the UK is now the first country in the world to approve an antiviral that can be taken at home for Covid-19.”
Read more: Potential game-changer in the Covid battle, FDA authorization sought for molnupiravir
“This will be a game-changer for the most vulnerable and the immunosuppressed, who will soon be able to receive the ground-breaking treatment,” he maintained.
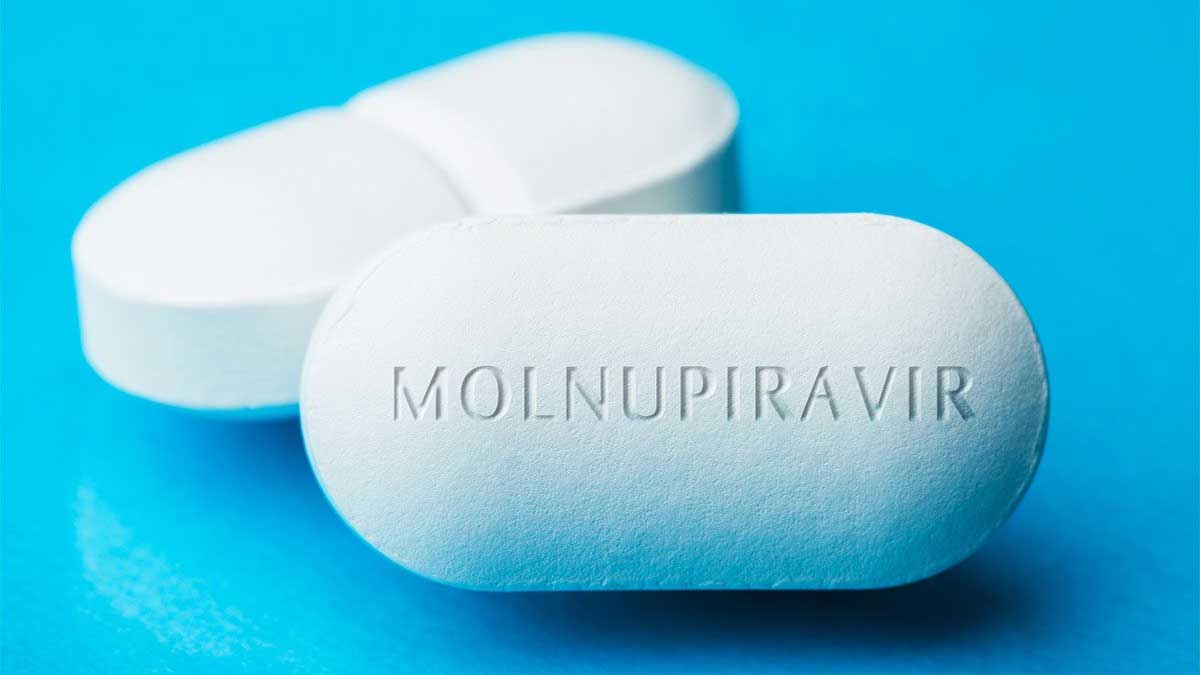
Molnupiravir is the antiviral approved by Britain. It works by reducing the ability of a virus to reproduce, thus slowing down the ailment.
Moreover, the Medicines and Healthcare products Regulatory Agency (MHRA) told the media that its trials had established it was “safe and effective at reducing the risk of hospitalization and death in people with mild to moderate Covid-19 who are at increased risk of developing severe disease”.
On the basis of clinical trial data, the results of the drug are most operational when taken in the initial stages of contagion and the MHRA commends that it can be used within five days of the start of indications.
Furthermore, the Covid pill has been certified for use in people who have at least one hazard factor for the emerging stark ailment, including obesity, diabetes, old age, and heart disease.
Besides this, Merck has before now made commitments with other governments, including the US, which is deliberate to purchase 1.7 million doses if molnupiravir is permitted by regulators.
The chief executive of MHRA June Raine named the pill “another therapeutic to add to our armory”.
“It is also the world’s first approved antiviral for this disease that can be taken by mouth rather than administered intravenously,” she added.
“This is important because it means it can be administered outside of a hospital setting.”
Initially, Molnupiravir was made as an inhibitor of influenza and respiratory syncytial virus, which are two other significant severe respiratory infections, by a team at Emory University in Atlanta, Georgia.